Science has always helped humanity to invent something new and do something extraordinarily beautiful. So, using the method of electroplating, modern society today is trying to copy the elements of nature: leaves, flowers, insects, beetles, and make real works of art out of them. And why not: such products will always be relevant and popular, since man has not yet been able to come up with better forms than nature did.
Let's try today to understand at least a little what electroforming is, what examples of electroforming we can meet in everyday life, and how it is used to create the most extraordinary beauty jewelry.
What is electroforming in plain language?
Electroplating - technology for making exact metal copies various objects, by the deposition of dissimilar metals on the model. In other words, we pick up an object that we want to copy down to the smallest detail. Further, through electrochemical influence and heterogeneous manipulations, we deposit a thin layer of metal on this object. In the next step, the model is separated from the coating. Everything.
The main purpose of the electroforming method is obtaining an exact metal copy of the model, reproducing the exact shape of an object, or applying a thin coating of metal. You most likely understand that if you pick up a leaf from any tree, then it is simply impossible to copy it to the smallest detail by its mechanical methods. Only a machine or a super accurate robot is capable of such feats. However, all the same, the computerized brain is not able to reproduce all the kinks, bulges and the smallest details.
Electroforming got such a romantic name for the reason that the metal deposited in the process of electrolysis "plastically exactly" reproduces the product, the imprint of which was in the form.
The beginning of this technology in Russia and throughout the world is associated with the name of B.S. Jacobi (1801-1874), who discovered the method of electroforming. Jacobi was born in Germany - here his name was Moritz Hermann. In 1835 he moved to Russia.
In 1836 was Jacobi B.S... invented an original design of a copper-zinc galvanic cell. October 4, 1838 B.S. Jacobi officially announced his invention in a letter to the Secretary of the Academy of Sciences P.N. Fuss to the city of St. Petersburg. The next day, the invention was immediately reported at a meeting of the Academy of Sciences. Attached to the letter was an electroplated copy of an engraving board depicting a two-headed eagle. Under the eagle there was an inscription made by the scientist's wife: "The great soars and overshadows the great."
Electroforming method gives the most unlimited copying possibilities, and, accordingly, for creativity. This method allows you to create documentary accurate:
- copies of bas-reliefs;
- coats of arms;
- coins;
- medals;
- memorial plaques;
- emblems;
- plates with inscriptions.
Also this technology widely used in restoration or creating interior items, sculptures, figurines, lamps, candlesticks, household items, souvenirs, etc.

Operating principle
Principle of operation this method is pretty simple. Consider the cover and copy processes.
Покрытие
An object, such as a spoon, fork, plate, coin, plastic, enamel, leaf from a tree, is placed in the bathroom with a chemical solution. Further, this entire system is connected to a power source and an electrochemical reaction is triggered, in which the metal from the solution (that is, the metal atoms present in the liquid) is deposited on the surface of the object. The longer the reaction is maintained, the thicker the sediment is obtained on the model. Then the process stops, the model is cooled and washed in running water from reagents. Everything is ready, the object is covered with a layer of metal.
Copying
If you are not going to coat an item, but completely copy without exposing him to any influences, then the path is slightly different.
- From the copied object or product (let's call it a model), first of all, an imprint is taken from both sides on low-melting metal, wax, plasticine or plaster, as, for example, an imprint of a key is made for copying.
- Further, these two half-copies of the prints are connected (glued) together, leaving a hole for pouring a model material such as wax - this design is called a casting mold.
- After that, liquid low-melting metal is poured into this form.
For the gilding of the domes of the Cathedral of Christ the Savior in Moscow, St. Isaac's Cathedral, Peter and Paul Cathedral and several others, an electroplating workshop with the participation of B.S. Jacobi spent 45 poods 32 pounds (750,2 kg) of gold.
- The mold is placed in a cold place to freeze the master model.
- To get the resulting master model, the casting mold is sawn into two halves so as not to damage the resulting copy in any way. Then it is degreased and subjected to copper plating in an electrolytic bath, that is, electroplating... To prevent metal from depositing on those sides of the mold where there is no print, they are covered with molten wax or paraffin using a brush.
- After the electroforming process, the fusible metal enclosed in a copper sheath is melted in boiling water, thus forming a matrix. The latter is filled with plaster or lead, and the copy is ready.

Advantages
Making copies of items by electroplating provides a number of advantages:
- high quality playback;
- the ability to manufacture products with a relief height of up to 20 mm;
- the possibility of finishing products from electroforming - giving a given color and covering with protective materials;
- low cost in comparison with other technologies - casting and embossing;
- the ability to manufacture both single items and large batches;
- high speed of production of electroforming and the possibility of a quick start of production;
- less weight of an electroformed product compared to a metal casting.
Home Electroplating Training From Scratch
To repeat the experiment at home, you will have to prepare well, but we warn you in advance that you will have to deal with toxic chemicals. Therefore, either leave the experiments for specialists, or conduct your experiments in a well-ventilated room with powerful hoodso as not to get poisoned.
Equipment
What equipment needs to be assembled:
- As the galvanic bath you can use any glass container of such a size that the object to be covered with metal fits freely in it.
- As the current source You can use a car battery or power supply with a voltage range of 6 to 12V.
- The model will cling to the conductor using copper wires... The wire diameter should be approximately 0,8 ... 1 mm.
- The current lead is usually made of copper rods, which are then in turn connected to the power source.
- It is necessary to constantly maintain a certain temperature in the bath with the reagent. For this you will need heater and cooler... As a heater, you can use a conventional boiler or an electric stove. Experts do not recommend using an open fire for heating, since it is difficult to control the temperature regime with an open fire, you can accidentally overheat the solution, which we do not need at all. In addition, there is a risk of spoiling an expensive gas stove with some poisonous solution. As a cooler, you can lower a soft hose into the container and connect it to a tap with running water.
- Thermometer for temperature control within 18 °… 25 ° C. It is advisable to use an alcohol device, as it measures temperature more accurately than any other electrical counterpart.
- During the reaction, the liquid will need to be constantly stirred. For this it is possible to use the usual aerator or circulator water for the aquarium.
- Respirator and gloves... Any chemical reagents are very bad friends with a person, therefore, it is imperative to observe safety precautions in order to protect yourself from getting chemistry on your hands and face with the help of protective equipment.
- Crockery... It is best to buy a set of chemical utensils (flasks, cups, boilers) at the market or bazaar. But if such an opportunity was not presented, then you can use any household glassware. You will also need glass bottles with a ground-in lid. They are essential for storing reagents and electrolytes.
- Measuring equipment... What you cannot do without is a balance, since you will have to measure the reagents with an accuracy of 1 gram. You will also need a liquid density meter, a voltmeter for voltage and an ammeter for current.

Preparing the solution
Next solution preparation... If you want to deposit copper on the surface of the plate, for example, for copper plating of leaves, then take 150-180 g of copper sulfate. It must be carefully mixed with 1 liter of clean (preferably distilled) water. Sulfuric acid with a density of 1,4-1,6 g / cm3 and a mass of 20-25 g is added to this chemistry to increase the electrical conductivity. To improve the quality of precipitated copper, alcohol in an amount of 8-10 g / l can be added.
Remember that the electrolyte should not contain any organic inclusions that adversely affect the operation of the solution.
Chemical compositions of electrolytes for nickel plating, silver plating, gilding, etc. differ from each other, but their composition necessarily contains acid, water and sulfates or nitrates of the applied metals.
Calculation of current density
Even dense coating of an object with metal (copper, nickel and other metals) obtained at a certain current value, which depends on two factors:
- surface area of the object;
- current density permissible for a given electrolyte.
Produced next calculation... For example, a model of an object has a total surface of 0,5 dm2. The permissible current density in the reaction should be 0,5 A / dm2. This means that the current should not exceed Imax = 0,5-0,5 = 0,25 (A).

Now we collect the circuit
We need to prepare two electrodes, which are called the cathode (negative potential electrode) and the anode (positive potential electrode).
In our scheme the cathode consists of three parts:
- the model to be copied;
- copper conductor;
- current lead.
The model is very rigidly fixed by hand on the conductor. This part, in turn, is hung on a current lead, which is mounted horizontally on a glass container. Further, using ordinary wires, the current lead is connected to the negative pole of the power source.
We collect anode... There should be two anode plates. Usually they are made of two thick copper plates, which are installed in the bath at a certain distance from each other, in parallel. The distance is chosen in such a way that a suspended model of the cathode is freely placed between the anode plates. However, you can make the anode in another way. Take one copper plate and fold it into a cylinder. At the same time, keep in mind that the volume inside the cylinder should be enough for the cathode to fit there without any problems, with all its suspensions. It is important that the objects to be coated face the anodes with their largest areas and are with them in approximately parallel planes.
Next anodes are attached to horizontal current leads... These crossbars must be provided with terminals for convenience and reliability of the connection. The wires holding the anode to the bar must be above the electrolyte level, especially if they are made of a different metal. Otherwise, in this place, the wire becomes thinner and burns out.
Anodes must be very thoroughly cleaned of oxides, dirt and grease, as well as items intended to be coated with metal.
An important condition for the success of this technology is purity... If a slight turbidity appears in the electrolyte or a precipitate forms, then the solution must be filtered. To do this, find an empty container of sufficient size. Wash her up. Dry. During this time, all the dirt in the solution will sit down. Carefully, without overturning the glass bathroom, pour the solution through a tube (as gasoline is drained from a car) or a cup (using the scooping method). You should not scoop up the thick from the bottom. It is better to simply dispose of it. However, the remains of the solution should not be flushed down the drain. Plumbers can then work with pipes, who simply burn their hands upon contact with your chemicals, and the work from such a solution will not thank you.

Electrical connection diagram
You can find the wiring diagram on the Internet without any problems. However, there is one simple rule to keep in mind. To obtain a good deposit, it is necessary that the proper amperage is maintained per unit surface area of the cathode during the reaction. To regulate the electrical circuit, a rheostat is used, which is introduced through the wires to the bath. And to know the voltage across the electrodes, a voltmeter is installed.
You could see examples of electroplating products in churches, museums, exhibitions. These are icons, emblems, plates with inscriptions.
Finish
Remember that an item removed from the bathtub, no matter how well it has been pre-polished, has a matte finish. To make it shine, it is polished with the finest chalk (tooth powder) using a cloth or rubberized circle.
Metal plating of non-metallic objects: beetles
To cover various insects, butterflies, beetles and other objects with metal, they must be prepared in an appropriate way. Insects soak in a 1,5% solution of mercuric chloride (mercury chloride II, a colorless crystalline water-soluble very toxic compound). Then they are dried, covered with a special varnish or a thin layer of wax. Then the entire surface must be made conductive, otherwise the metal will not lie down. For this, the beetle is smeared with a liquid gruel of graphite diluted with alcohol or vodka with a brush. After drying, the excess graphite is removed.
After that the insect suspended on several thin copper wires with a diameter of 0,1-0,2 mm and placed in an electroplating bath. To eliminate buoyancy in the electrolyte, a butterfly or bug is attached with paraffin to a weight. It could be glass or a piece of plastic. This process usually takes several hours. The thickness of the coating can vary from 0,1 to 2 mm, depending on the exposure time.

Is it possible to make jewelry using the method of electroplating at home?
There is only one answer to this question: yes, you can do everything, but you will have to "strain yourself" well. There are several reasons for this:
- In principle, everything you need for experiments can be bought today in offices selling chemical reagents. But there is one "but". In Russia, the process of acquiring chemistry has recently become a very difficult task. The buyer of reagents is required to present a power of attorney, some kind of extract from a permit document that, they say, this person can engage in the relevant activity, payment is usually non-cash and other difficulties. To solve this problem, you can go a simpler way and contact household stores, markets, service stations or comrades.
- In addition to reagents, it is necessary to purchase normal equipment... You can't do with an ordinary can. At a minimum, you will need a clean, well-made aquarium. Otherwise, the reagent will be on the table, legs, floor, wherever possible. And this is not entirely safe for health.
- You will find a huge number of descriptions of electroforming algorithms on the Internet and in books, however mistakes and lack of experience have not been canceled... Before setting up production, you will have to spend more than one hour conjuring up to understand: which current is better to choose, how to properly clean the surface of products from dirt, how to make forms correctly, etc.
- Remember one council: if you are going to silver metals or to cover with gold any items made of silver, then it is better to hone your skills on ordinary copper or nickel. Don't go straight to expensive metals. You just throw money down the drain, and nothing really works out.
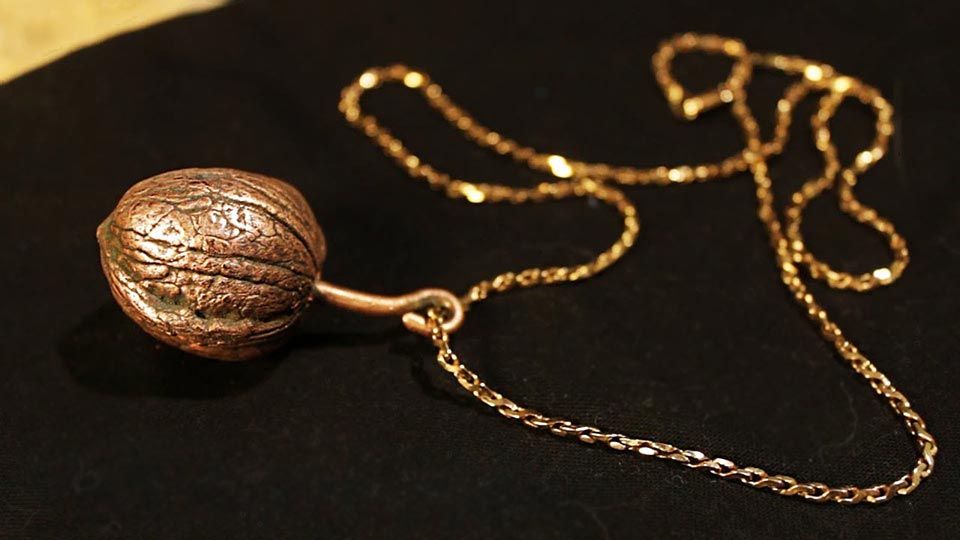
Master class for making jewelry using the technique of electroplating at home
Most often, we are interested in the technique of electroplating from the point of view of making various jewelry, both bijouterie and jewelry. Let's figure it out with a simple example, how at home you can make an ornament from leaves.
- To begin with, it is necessary to remove prints from fresh leaves, for which a wax composition is poured into a mold of thick paper, Then they allow it to cool down almost to complete hardening, but in such a way that its surface is elastic. Then leaves are placed on the surface of the wax and pressed with glass. When the glass is removed, a clear print is left on the wax composition.
- After the wax has completely hardened, a conductive coating must be prepared. The imprint is carefully graphitized with a soft brush to create an electrically conductive layer. The conductive layer can be applied by reducing certain metals (silver, copper, nickel) or by mechanical means: rubbing a form of flaky graphite into the surface with a soft hair brush or coating with graphite varnish.
- Next, you need to measure the leaves in order to determine the area of the deposited surface, calculate the current density and resistance of the rheostat.
- After that, if the bathroom has already been used at least once, it is necessary to prepare the current leads. They are carefully cleaned with sandpaper from various oxides, dirt, wiped with a cloth and washed in running water.
- Don't forget to prepare the anode.
- The process of heating the bath is started using a heater or a boiler.
- Further, having installed the conductors on the form, the load (cathode) is suspended and lowered into the galvanic bath.
- The reaction process starts.
- After applying a sufficient layer of surface metal, the unit is turned off.
- Beauty is taken out of the bath, disconnected from the conductors, washed in pure distilled water and polished.

So, you are probably confused by reading this article. It's clear business. In order to reproduce this entire algorithm, a sufficient amount of knowledge from many branches of science and technology is required. Here you cannot do without preparation. However, everything comes with experience, great scientists are not born, but become, overcoming huge heaps of books, visiting many laboratories, undergoing attempts and failures. Therefore, if you want to experiment or just play with the great chemist, then do not be intimidated by smart words and a long list of works, but act, and soon the result will appear on the horizon.









